296 couples were referred for PGT because the female (55.1%; 163/296) or male (44.9%; 133/296) partner was a nonhomologous carrier. Table 1 summarizes the translocations tested, the age of the carrier parents at the time of testing, the use of repeat cycles, the biopsy specimens retested, and the rate of inconclusive testing (due to insufficient DNA or uninformative SNP data).
Table 2 summarizes the average number of blastocysts analyzed per cycle for rob(13;14), rob(14;21) and all combined robs. There were fewer blastocysts when maternal compared to paternal (mean 4.60 vs. 5.49 per IVF cycle, respectively), a deficiency largely associated with rob (14;13). This does not appear to be attributable to an age-related decline in female fertility. There was no significant difference between the age of female carriers and women with a carrier partner (average 34.84 years versus average 34.81 years, P= 0.873).
Isolation products in blastocysts with successful analysis are summarized in Table 3. Male carriers were significantly more likely to have blastocysts with balanced chromosomal complement (alternating segregation). The balanced rate for male carriers was 84.8% versus 62.8% for female carriers.P< 0.00001). Subset analysis restricted to rob(14;13) and rob(14;21) showed a higher frequency of balanced products for male carriers for each of these more common deletions.
Table 3 also shows the breakdown of monosomy versus trisomy in isolates interpreted as contiguous. Overall, the proportion of blastocysts that were monosomy was significantly lower for male carriers (152/60, 39.5%) than for female carriers (396/218, 55.1%) (test for two independent proportions, P= 0.001). However, this difference depended on the specific type of theft. For rob(13;14), the same pattern was observed. Less monosomy for chromosomes 13 or 14 was observed for male carriers (36/103, 35.0%) compared to monosomy for chromosomes 13 or 14 for female carriers (140/251, 55.8%).P= 0.0004). For rob(14;21), no such difference was evident with monosomy of chromosome 14 or 21 for male carriers (12.24, 50%) versus monosomy of chromosome 14 or 21 for female carriers (50.93, 53.8%).P= 0.74).
There was also evidence for nonrandom segregation products for 21 blastocysts with chromosomal complements consistent with 3:0 segregation (16 with double trisomy and 5 with double monosomy). For maternal carriers, 16 cases showed apparent 3:0 segregation including double trisomy and one double monosomy. In contrast, no double trisomy was observed for paternal carriers, but four double monosomies were detected (Fisher’s exact test, P< 0.05).
In six blastocysts (0.7%), there was a combination of one trisomy and one monosomy, inconsistent with the eight possible segregation products that can arise during segregation of a steal. We refer to them as “unconventional”. All six products were maternal carriers, five included rob(13;14) with trisomy 13 and monosomy 14, and one included rob(14;21) with trisomy 14 and monosomy 21. There was only one case of UPD (heterodisomy). observed in our dataset. This case was about a man who was an armed robber (15;21) with a grave [15]Blastocyst sample mat x2 htz.
To provide robust data for genetic counseling for individuals with rob(13;14) and rob(14;21), we combined our observations on the odds of a balanced segregation with those of two other recent articles (Table 4). We then calculated the probability of at least one normal blastocyst using a binomial calculator, assuming that each embryo was completely independent of the others from the same patient. These observations indicated that for an average referral with four to six blastocysts available (Table 2), PGT should significantly increase the chance of selecting a balanced embryo. These calculations did not include sporadic chromosomal abnormalities unrelated to theft.
To explore potential associations between carrier age and segregation patterns, data from male and female carriers were analyzed separately, and data were grouped into 3-year age ranges. Figure 1A shows that there is no trend in unbalanced segregation rates for maternal carriers in different maternal age groups (Armitage-Cochran test, P= 0.58, slope = 0.006). Because maternal and paternal age are correlated, the lack of an observed age association for paternal age was expected and observed.P= 0.23, slope = 0.014) (Figure 1B). To further search for evidence of a maternal age effect, we considered only the 3:0 segregation of female carriers. For 17 cases (16 cycles) with 3:0 segregation in blastocysts from female carriers, the mean maternal age at the time of testing was 34.6 years, compared with 34.8 years for female carriers with other types of segregation (Mann-Whitney test, P= 0.624).
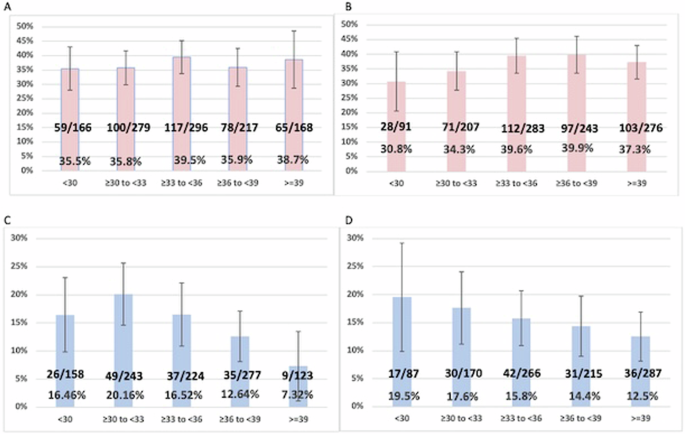
x-axis, age group; y-axis ratio (%) with unbalanced separation. A Maternal age for female carriers b Father’s age for maternal carriers. C Maternal age for male carriers d Father’s age for male carriers. Vertical lines show 95% confidence intervals.
For paternal carriers, there was a trend toward lower rates of unbalanced segregation with increasing parental age (Fig. 1C, D). For father’s age, this was not statistically significant (P= 0.052, slope -0.017) but for mother’s age, a stronger trend was observed (P= 0.0035, slope -0.026) which was significantly different from linearity and indicated a possible non-linear relationship.
To assess whether unbalanced deletions are associated with increased levels of other chromosomal abnormalities (interchromosomal effect) [10, 21]or decreased levels due to reduced viability of embryos with multiple chromosomal imbalances, we assessed the rate of sporadic chromosomal abnormalities (ie, unrelated to spoliation). When the fetus had unbalanced abduction, the rate of sporadic malformations was 38.7%. For fetuses balanced or normal for rob chromosomes, the rate of sporadic abnormalities was 42.8%. The difference between these two rates was not statistically significant (P= 0.086). For a maternal age-matched control population (excluding oocyte donors) who were not translocation carriers, the rate of sporadic abnormalities was 44.1%. These data do not completely rule out an interchromosomal effect involving specific chromosomes (eg, acrocentrics not involved in translocation).
#Chromosomal #isolation #human #nonhomologous #Robertsonian #translocations #insights #preimplantation #genetic #testing #European #Journal #Human #Genetics